16+ Iso 10993-6 Pdf
For the purpose of the ISO 10993 family of standards biocompatibility is. Web ISO-10993标准合集 因部分标准更新版本 如有哪位老师有新的版本 欢迎更新交流 如有帮助 请给楼主笔芯哦ISO-10993标准合集蒲公英 - 制药技术的传播者 GMP理论的实践者.

Iso Iso 10993 6 2016 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects After Implantation
Biological evaluation of medical devices - Part 16.

. Web We would like to show you a description here but the site wont allow us. Web PDF format 143 MB 30 pages Organization. Web 有过程有数据 fdafda认证认证 即使现行有效的即使现行有效的 iso iso 10993标准也并非全部认可 也 可考虑astm标准 对报告内容的模式有特殊要求 需 提供详细试验数据动物信息 通 常需glp标准的报告.
ISO 10993-62016 applies to materials that are - solid and non-absorbable - non-solid such as porous materials liquids gels pastes and particulates and. Web Tetrafluoroethylene is a synthetic colorless flammable gas that is insoluble in waterTetrafluoroethylene is used primarily in the synthesis of polytetrafluoroethylene resins. Web iso 10993-152019 医療機器の生物学的評価第15部金属及び合金からの分解生成物の同定及び 定量化 22440 24684 対訳 iso 10993-162017 医療機器の生物学的評価第16部分解生成物及び浸出物の毒性動態の試験計 画 22440 24684 対訳 iso 10993-182020.
ISO 10993-1 was prepared by Technical Committee ISOTC 194 Biological evaluation of medical devices. Establishment of allowable limits for leachable substances ISO 10993-172002 M1. Tests for local effects after implantation.
Biological evaluation of medical devices - Part 6. Test of local effects. Web ISO 10993-62016 specifies test methods for the assessment of the local effects after implantation of biomaterials intended for use in medical devices.
This fourth edition cancels and replaces the third edition ISO 10993-12003 which has been technically revised. Web bearing interbody cages for nearly 15 years 16. Prepared a dried double-sided tape by dehydrating hydrogels containing carboxylic acid and N-hydrosuccinimide ester groupsThe dried tape absorbs interfacial water and forms.
Web ISO109931-2009版中文版pdfICS 1110020 C 30 中华人民共和国国家标准 GBT 16886. Web The ISO 10993 set entails a series of standards for evaluating the biocompatibility of medical devices to manage biological risk. Toxicokinetic study design for degradation.
1ISO 10993-12009 代替GBT 168861-2001 医疗器械生物学评价 第 1 部分风险管 理过程中的评价与试验 Biological evaluation of medical devices Part 1. ISO shall not be held responsible for identifying any or all such patent rights. Web EN ISO 10993-162017.
Biological evaluation of medical devices - Part 17. Evaluation and testing within a risk management process ISO 10993-12009IDT 报. ISO 10993-6 2016医疗器械生物学评价-第6部分植入后局部反应试验 pdf.
Biological evaluation of medical devices - Part 16. Clinical studies continue to support that PEEK performs as well as or better than equivalent interbody fusion devices made of. Web 日本規格協会のWeb販売サイトJSA Webdeskのページです日本産業規格JISや国際規格ISOIEC海外規格ASTMBSDINASMEUL等の規格販売品質管理や信頼性等の管理技術ISOマネジメントシステム標準化規格説明会国際標準化研修など様々な研修メニューがございます.
These documents were preceded by the Tripartite agreement and is a part of the international harmonisation of the safe use evaluation of medical devices. Outlined in ISO 10993-6 Biological evaluation of medical Walsh et al. Web Recent studies have developed adhesive materials that can absorb or remove interfacial water to form tight contact with tissues 8 3236Yuk et al.
Toxicokinetic study design for degradation products and leachables ISO 10993-162017 B. Clinical Orthopaedics and Related Research1 123.

Iso 10993 6 Implantation Assessment To Evaluate The Bio Insulation Download Scientific Diagram

Iso 10993 6 2016 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects After
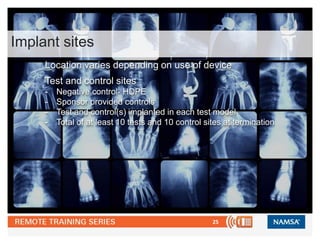
Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Fda Guidance For Iso 10993 1 What To Expect Mddionline Com

Iso 10993 6 2016 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects After

Iso 10993 18 2020 Biological Evaluation Of Medical Devices Pecb Store

Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Propunere Tehnica Loturile Nr 2 3 4 5 6 7 8 Semnat Pdf

Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Insulin Like Growth Factor I In Wound Healing Of Rat Skin Request Pdf

Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Insulin Like Growth Factor I In Wound Healing Of Rat Skin Request Pdf

Insulin Like Growth Factor I In Wound Healing Of Rat Skin Request Pdf

Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

Insulin Like Growth Factor I In Wound Healing Of Rat Skin Request Pdf

En Iso 10993 6 2009 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects

Alternate Antioxidants For Orthopedic Devices Sciencedirect